Slide Preparation of Encarsia specimens
Section Hymenoptera
The identification of Encarsia requires high-quality microscopic slides because their taxonomy is still primarily relies on morphological characters that can only be seen in a compound microscope. Despite their importance, the identification of many species is challenging because of their small size necessitating laborious slide-mounting for taxonomic study and the poor condition of most of the early (and some recent) type material.
The method described below is based on Noyes (1982) For specimens that were treated with destruction-free DNA extraction protocols, steps 1 and 4-13 can be omitted. In that case the wings are removed after step 19.
- Attach specimen with ventral surface to a small piece of card board with a very small drop of water soluble glue. Wings must remain free of glue. Specimens stored in ethanol have to be dried first by placing them on a tissue for a short time.
- Write the sample/specimen number on the card board.

- Glue labels on the slide and write the sample/specimen number on one of the labels. The labels should be made of heavy white card board (1-1.5 mm).
- Remove wings with an insect pin. This should be done carefully to avoid removing diagnostically important setae from the submarginal and marginal veins and is best achieved by moving the wings at the point where they are attached to the body. Preferably the fore wing comes off with the tegula attached.
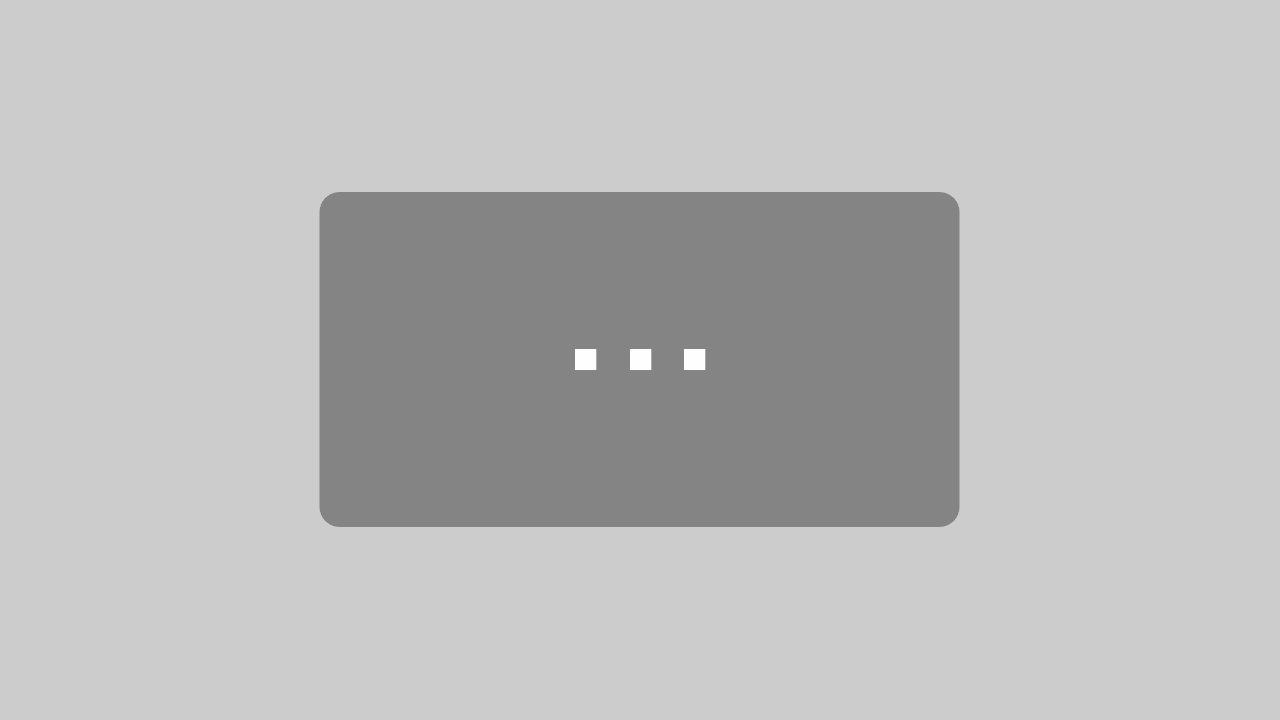
- Put a small drop of Canada balsam at the location of the slide which is designated for the wings. (Note: wings, body, and head should be oriented upside down so that the specimen is in upright position when examined with a compound microscope). Transfer wings to a drop of Canada balsam and arrange them properly. Leave the slide on a hot plate (60° C) for several hours to fix their position.
- Add a drop of water to the specimen on the card board. After a short while the specimen will float on top of the drop of water.
- Mazerate specimen in 10% KOH, either by heating in an Eppendorf tube for 5-7 minutes at 97° C using a block heater, or by leaving in an excavated block (covered with lid) at room temperature for about 12-24 hours.
- Remove the KOH with a pipette. For the following steps, the excavated block should be covered.
- Add 5 drops of glacial acetic acid and leave for 2 minutes.
- Remove the acetic acid and add 5 drops of distilled water. Leave for at least 15-30 minutes, longer (e.g. 2 hours) is better and helps to avoid collapsing at later steps in the procedure (in particular if specimen was preserved in ethanol).
- Add 5 drops of 70% ethanol and leave for 10 min.
- Remove liquid and add 5 drops of 70% ethanol. Leave for 10 minutes.
- Add 5 drops of absolute ethanol and leave for 10 minutes.
- Remove liquid and add a few drops of absolute ethanol so that specimen is just covered. Leave for 10 minutes.
- Add 3 drops of clove oil and leave excavated block uncovered for 20 minutes, to allow the ethanol to evaporate.
- If the specimen was not preserved in ethanol, move directly to step 19, otherwise add 3 drops of a mixture of 60% clove oil and 40% Canada balsam. Leave for 10 minutes.
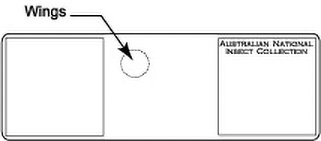
- Place three drops of Canada balsam on the slide at the appropriate locations for body, antennae, and head.
- Transfer specimen from clove oil (or clove oil/Canada balsam mixture if specimen was preserved in ethanol) to bottom left position with drop of Canada balsam that is designated for the body.
- Separate the head from the body with insect pin. This is easiest if the specimen is positioned on its side.
- Carefully transfer the head (with antennae attached) with an insect pin to the top right drop of Canada balsam.
- Detach the antennae from the head with ab insect pin. This is easiest if the head is in upright position and the outermost tip of the pin is used to remove the antenna at its very base. The radicle should be attached to the antenna and not to the head.
- Carefully transfer head with insect pin to the bottom right drop of Canady balsam.
- Arrange the body so that the legs are spread and the body is not tilted.
- After all parts have been arranged put the slide on a hot plate (60° C) for about 24 hours.
- Add a small drop of Canada balsam to each of the four positions and cover with round cover slips (6mm diameter). Ensure that the cover slips are horizontal.
- Place the slide immediately on a hot plate (60° C) for several hours.
- Store the slide in an oven for several weeks or until Canada balsam is sufficiently hardened.
- The slide should be labelled with locality information on the left and species identification on the right side.

Reference
Noyes, J.S. (1982) Collecting and preserving chalcid wasps (Hymenoptera: Chalcidoidea). Journal of Natural History 16, 315-334.