Phylogeny of the subfamily Coccophaginae (Hymenoptera, Aphelinidae)
Section Hymenoptera
Coccophaginae are minute parasitic wasps found world-wide. They primarily attack the sessile nymphs of Sternorrhyncha, including Aleyrodidae (whiteflies) and Diaspididae (armoured scale insects). The Coccophaginae are the largest subfamily of the Chalcidoidea family Aphelinidae with over 700 described species.

Slide mount of a female specimen of Encarsia whittieri Girault.
Encarsia is the largest genus of Coccophaginae and of the family Aphelinidae, currently containing almost 400 described species. Species of the genus have been of particular interest to economic entomologists because several of them have been used, or are currently being used, successfully for pest control.
Some Encarsia species appear to be extremely host-specific, probably the most important trait for an acceptable and effective agent. They are also of considerable biological interest in that most species for which the biology is known display heteronomous biologies, with males developing as hyperparasitoids on females of their own or other species.
Identification of Encarsia species
The identification of Encarsia species is often difficult because of their small size and the necessity to prepare slide mounts. New species are being continuously added and there is evidence for the presence of complexes of cryptic species within several of these described species. Many Encarsia species have a very wide or even cosmopolitan distribution, complicating taxonomic revisions on a local scale. Males are often very difficult to identify without accompanying females. In several species males are not known.
Slide prepartion of Encarsia specimens
(after Noyes, 1982, with modifications)
- Attach specimen with ventral surface to a small piece of card board with a very small drop of water soluble glue. Wings must remain free of glue. Specimens stored in ethanol have to be dried first by placing them for a short time on a tissue.
- Write sample/specimen number on card board.
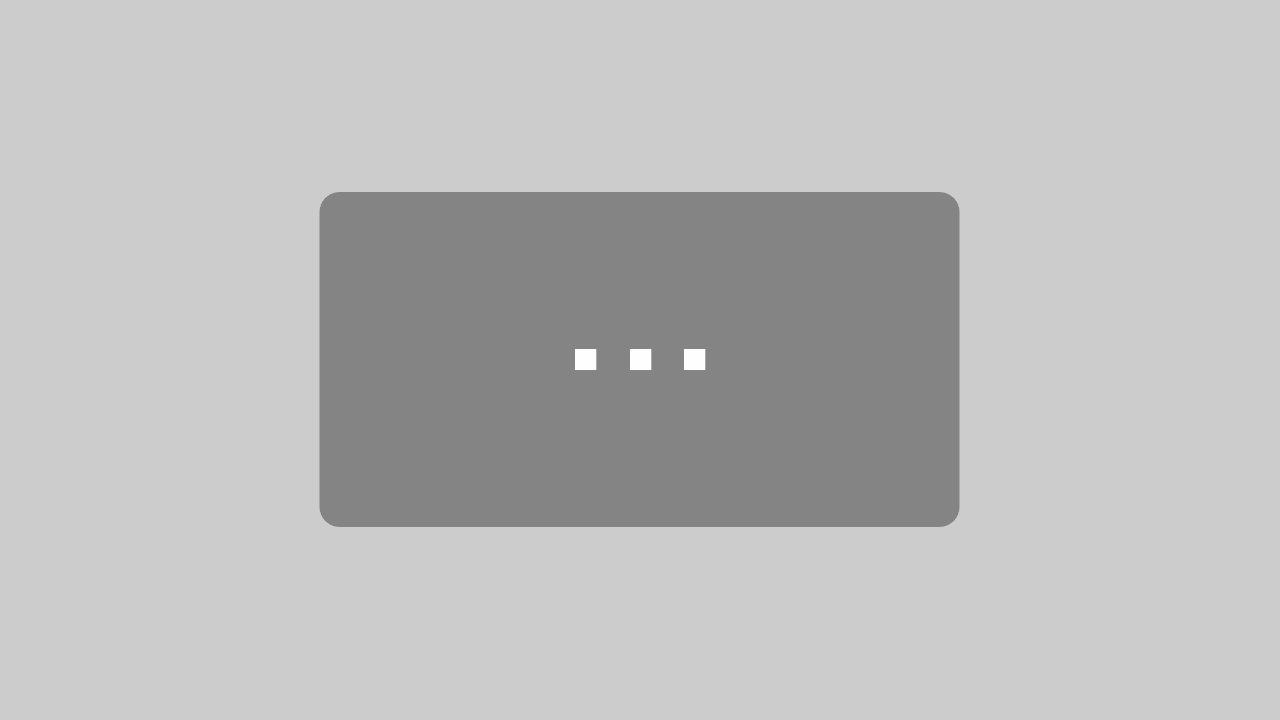
 Glue labels on the slide and write sample/specimen number on label. The labels should be made of 1-1.5 mm thick white card board
Glue labels on the slide and write sample/specimen number on label. The labels should be made of 1-1.5 mm thick white card board- Remove wings with insect pin. This should be done carefully to avoid removing diagnostically important setae from the submarginal and marginal veins and is best achieved by moving the wings at the point where they are attached to the body. Preferably the fore wing comes off with the tegula attached.
- Put a small drop of thin Canada balsam at the location of the slide which is designated for the wings. (Note: wings, body, and head should be oriented upside down so that the specimen is in upright position when examined with a compound microscope).
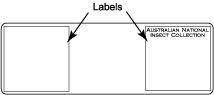
 Transfer wings to drop of Canada balsam and arrange them. Leave slide on a hot plate (60° C) for several hours to fix their position.
Transfer wings to drop of Canada balsam and arrange them. Leave slide on a hot plate (60° C) for several hours to fix their position.- Add drop of water to the specimen glued on the piece of card board. After a short while the specimen will float on the drop of water.
- Mazerate specimen in 10% KOH, either by heating in an Eppendorf tube for 5-7 minutes at 97° C using a block heater, or by leaving in an excavated block (covered with lid) for about 12-24 hours at room temperature.
- Remove KOH with pipette. For the following steps, excavated block should be covered with lid.
- Add 5 drops of glacial acetic acid and leave for 2 minutes
- Remove acetic acid and add 5 drops of distilled water. Leave for at least 30 minutes, longer (e.g. 2 hours) is better and helps to avoid collapsing at later steps in the procedure (in particular if specimen was preserved in ethanol).
- Add 5 drops of 70% ethanol and leave for 10 min.
- Remove liquid and add 5 drops of 70% ethanol. Leave for 10 minutes.
- Add 5 drops of absolute ethanol and leave for 10 minutes.
- Remove liquid and add a few drops of absolute ethanol so that specimen is just covered. Leave for 10 minutes.
- Add 3 drops of clove oil and leave excavated block uncovered for 20 minutes.
- If specimen was not preserved in ethanol move directly to step 19, otherwise add 3 drops of a mixture of 60% clove oil and 40% Canada balsam. Leave for 10 minutes.
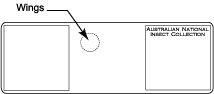
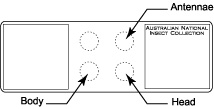 Place three drops of thick Canada balsam on the slide at the appropriate locations for body, antennae, and head.
Place three drops of thick Canada balsam on the slide at the appropriate locations for body, antennae, and head.- Transfer specimen from clove oil (or clove oil/Canada balsam mixture if specimen was preserved in ethanol) to bottom left drop of Canada balsam which is designated for the body.
- Separate head from body with insect pin. This is easiest if the specimen is lying on its side.
- Carefully transfer head (with antennae attached) with insect pin to top right drop of Canada balsam.
- Detach antenna from head with insect pin. This is easiest if the head is in upright position and the outermost tip of the pin is used to remove the antenna at its very base. The radicle should be attached to the antenna and not to the head.
- Carefully transfer head with insect pin to bottom right drop of balsam.
- Arrange body so that legs are spread and body is not tilted.
- After all parts have been arranged put slide on hot plate (60° C) for about 24 hours.
- Add a small drop of thin Canada balsam to each of the four positions and cover with round cover slips (6mm diameter). Care should be taken that the cover slips are horizontal.
- Place immediately on a hot plate (60° C) for several hours.
 Store in an oven for several weeks or until Canada balsam is sufficiently hardened.
Store in an oven for several weeks or until Canada balsam is sufficiently hardened.- The slide should be labelled with locality information of the left and species identification of the right side.
Project collaborators
Dr Andrew Polaszek, Entomology Department, Natural History Museum Cromwell Road London SW7 5BD, UK.
Dr Paolo A. Pedata, Maurilia Monti, Istituto CNR per la Protezione delle Piante Sezione di Portici Via Università 133 80055 Portici (Napoli), Italy.
Publications
Schmidt, S., De Barro, P. & Jamieson, L. (2011) Parasitoids of the Australian citrus whitefly, Orchamoplatus citri (Takahashi) (Hemiptera, Aleyrodidae), with description of a new Eretmocerus species (Hymenoptera, Aphelinidae). Zootaxa 2873: 27–34. DOI:10.5281/ZENODO.200548
Schmidt, S. & Polaszek, A. (2007). The Australian species of Encarsia Förster (Hymenoptera, Chalcidoidea: Aphelinidae), parasitoids of whiteflies (Hemiptera, Sternorrhyncha: Aleyrodidae) and armoured scale insects (Hemiptera, Coccoidea: Diaspididae). Journal of Natural History 41(33-36): 2099–2265. DOI: 10.1080/00222930701550766
Schmidt, S. & Polaszek, A. (2007). Encarsia or Encarsiella? – redefining generic limits based on morphological and molecular evidence (Hymenoptera, Aphelinidae). Systematic Entomology 32: 81-94. DOI: 10.1111/j.1365-3113.2006.00364.x
Schmidt, S., Driver, F. & De Barro, P. (2006) The phylogenetic characteristics of three different 28S rRNA gene regions in Encarsia (Insecta, Hymenoptera, Aphelinidae). Organisms, Diversity & Evolution 6: 127-139. DOI: 10.1016/j.ode.2005.07.002
Schmidt, S., Naumann, I.D. & P. De Barro (2001). Encarsia species (Hymenoptera: Aphelinidae) of Australia and the Pacific Islands attacking Bemisia tabaci and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) – A pictorial key and descriptions of four new species. Bulletin of Entomological Research 91: 369-387. DOI:10.1079/BER2001112
Schmidt, S. (2001) Encarsia Online – Encarsia species of Australia and the Pacific Islands attacking Bemisia tabaci and Trialeurodes vaporariorum. CSIRO Entomology, Canberra, Australia